Smoke Plumes and Air Pollution
What is a Plume?
A plume is a column of liquid, gas, or dust, moving through another. In general we use the term plume to describe things like smoke coming out of a chimney or steam from a smokestack at a power plant. A plume could also be rain cloud, a snow cloud, and even your breath!
What is Air Pollution?
Air pollution is chemicals, small bits of dust, or waste from living things that float in the air. Air pollution can cause harm or discomfort to humans or other living things in our neighborhoods, state, country, and all around the earth. Air pollution is generated from both human activities and natural occurrences. Human sources are mostly related to burning of fossil fuels (such as factory exhaust and car exhaust) but also include household and agricultural pollution. Natural sources include dust generated by wind, cattle or sheep passing gas, wildfires, and volcanic activities. In the Bay Area, the majority of the air pollution comes from man-made sources, such as exhaust pipes of cars and smokestacks from factories and power plants. The following video shows plumes from two smokestacks.


Plume Spreader
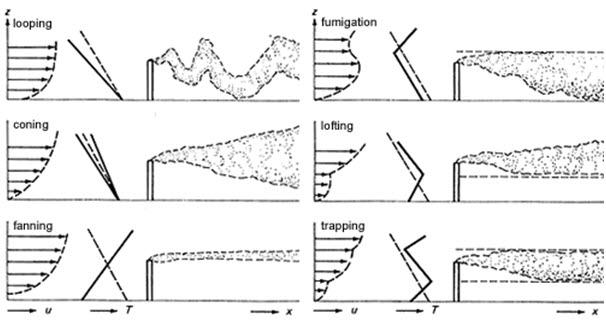
To understand how natural and man-made pollution affects the surrounding environment, atmospheric scientists are interested in how plumes spread in the air. The pictures to the left show movement and concentration of a plume away from the smokestack.
The plume becomes wider as the pollutant travels further away from the source. The spreading is called dispersion. The plume “disperses” when the edges of the plume mix with the surrounding air. The plume has lower concentration farther away from the source.
Notice that the plume rises after it leaves the stack. Buoyancy is a force generated by a difference in density. When something is lighter, it “floats” on top of things that are thicker and heavier. The buoyancy of the hotter, lighter air from the smokestack pushes it above the colder heavier surrounding air. Buoyancy determines how much the plume rises and spreads in the atmosphere. Rapid mixing of the smoke as it moves higher is referred to as convection.
Atmospheric Stability
Atmospheric stability, the resistance of the atmosphere to mixing, is a term used to describe how much upward and downward mixing of a plume will occur in the atmosphere.
This upward and downward mixing is influenced by heating and cooling at the ground. Normally the air temperature gets colder as you go further away from the ground. During the daytime, the land surface is heated by the sun and this warms the air near the ground. This warmer air is less dense (lighter), so it rises, creating mixing in the atmosphere. This is called an unstable condition. Large updrafts and downdrafts create lots of mixing.
At night, the ground surface cools. This cools the air near the ground, which is denser (heavier) than the air above, so the air stays near the ground and does not want to mix, often trapping pollution near the ground. This is a stable condition. Extremely stable atmospheric conditions occur when the temperature actually increases with height; this is called an inversion.
Inversion layers can occur at the ground surface or at the top of the boundary layer. Inversion layers serve as a “capping lid” over the boundary layer – they are visible as the top of the smog layer over an urban area. The inversion keeps the pollution near the ground and prevents it from rising any higher.
Air quality engineering focuses on the effects of atmospheric stability on urban air pollution. For instance, during the early morning, upward mixing of plumes is prevented by the inversion layer. This may raise pollutant concentrations at the ground to hazardous levels. In the winter when heating from the sun is weak and the ground is cold, an inversion forms overnight and traps pollution near the ground. Accumulation of pollutants may result in severe air pollution episodes, sometimes lasting several days. Here's a local news story about local air pollution. To help reduce air pollution throughout the Bay Area, the Bay Area Air Quality District started the Spare the Air Day Program in 1991.
Natural Plume in the Bay Area: Angel Island Fire (October 12-13, 2008)
Wildfire is a natural source of air pollution that generates smoke and other pollutants. Similar to the smokestack plumes discussed above, smoke generated from the fire rises upward due to buoyancy generated by the hot fire. Big wildfires generate heat-driven winds that make the fire spread faster and make firefighting efforts more difficult.
Here is the webcam footage of the Bay from the following day after the webcam was restored. The next day, the smoke moved horizontally to the south (leftward) but barely spread upwards. As the wind changed direction, the smoke changed direction but still did not mix upward/downward very much. The smoke was trapped in the lower atmosphere due to an inversion layer. As the day progressed and the ground surface got warmer later in the day, the inversion layer was destroyed, so the plume could spread more.
Here is the webcam footage of the Bay from the following day after the webcam was restored. The next day, the smoke moved horizontally to the south (leftward) but barely spread vertically. As the wind changed direction, the smoke changed direction but vertical mixing remained limited. The smoke was trapped in the atmosphere due to the inversion produced by cooling throughout the night. As the day progressed and the surface got warmer later in the day, the surface inversion was lifted up, and the plume spread vertically.
Bibliography
- Stull, R.B.. Meteorology for Scientists and Engineers. 2nd ed. Brooks Cole, 1999.
- Jacob, Daniel J.. Introduction to Atmospheric Chemistry. Princeton University Press, 1999.
*“The source of the smokestack/wind speed material is the COMET® Website at http://meted.ucar.edu/ of the University Corporation for Atmospheric Research (UCAR), sponsored in part through cooperative agreement(s) with the National Oceanic and Atmospheric Administration (NOAA), U.S. Department of Commerce (DOC). ©1997-2013 University Corporation for Atmospheric Research. All Rights Reserved.
This website is part of a design project from CEE 105, Applied Fluid Mechanics. Authors: Lauren Goodfriend, David Hong, Albina Ouzon (Edits by Dr. Chow). For more information visit this page.